- 1Laboratory of Neuroepigenetics, Medical Faculty of the University of Zurich and Department of Health Science and Technology of the Swiss Federal Institute of Technology, Neuroscience Center Zurich, Zurich, Switzerland
- 2Swiss Federal Institute of Technology Zurich, Zurich, Switzerland
Prolonged periods of social isolation can have detrimental effects on the physiology and behavior of exposed individuals in humans and animal models. This involves complex molecular mechanisms across tissues in the body which remain partly identified. This review discusses the biology of social isolation and describes the acute and lasting effects of prolonged periods of social isolation with a focus on the molecular events leading to behavioral alterations. We highlight the role of epigenetic mechanisms and non-coding RNA in the control of gene expression as a response to social isolation, and the consequences for behavior. Considering the use of strict quarantine during epidemics, like currently with COVID-19, we provide a cautionary tale on the indiscriminate implementation of such form of social isolation and its potential damaging and lasting effects in mental health.
Introduction
Social behavior is a major life component of many organisms. Proper behavior in response to environmental conditions and signals is critical for development, reproduction, and survival (Chen and Hong, 2018). In mammals, social behavior is exquisitely regulated by brain mechanisms that depend on the control of gene expression during development and in response to life experiences (Cole et al., 2007; Zayed and Robinson, 2012; Chen and Hong, 2018). Accumulating evidence suggests that chromatin-based processes and molecular mechanisms including DNA methylation, non-coding RNA (ncRNA) and transcription factors play critical roles in the control of gene regulatory networks that establish and modulate social behavior (Yao et al., 2016; Hwang et al., 2017; Bludau et al., 2019; Seebacher and Krause, 2019; Nord and West, 2020). However today, how the modulation of gene expression can shape behavioral responses to experiences, such as social isolation, during early postnatal development and in adult life is poorly understood (Hilakivi et al., 1989; Weiss et al., 2004; Zelikowsky et al., 2018). Particularly, when social interactions are perturbed by social isolation, a special condition during periods of pandemics like the one we are currently going through, this can directly impact mental health and have consequences throughout life.
This review provides a comprehensive overview of the effects of prolonged periods of social isolation on the body and describes the known molecular events leading to behavioral alterations. We review the current evidence linking social isolation with changes in gene expression in the brain, and the effects on regulators of genome activity such as epigenetic modifiers, ncRNA and transcription factors. Direct functional evidence supporting the role of miRNAs and long ncRNAs (lncRNAs) as modulators of social behavior and their link to behavioral abnormalities observed during and after prolonged social isolation are discussed. Finally, we reflect on the effects that prolonged social isolation, such as observed during strict quarantine in epidemics, can have on mental health and discuss interventions that may help to ameliorate their burden.
Effects of Social Isolation in Humans
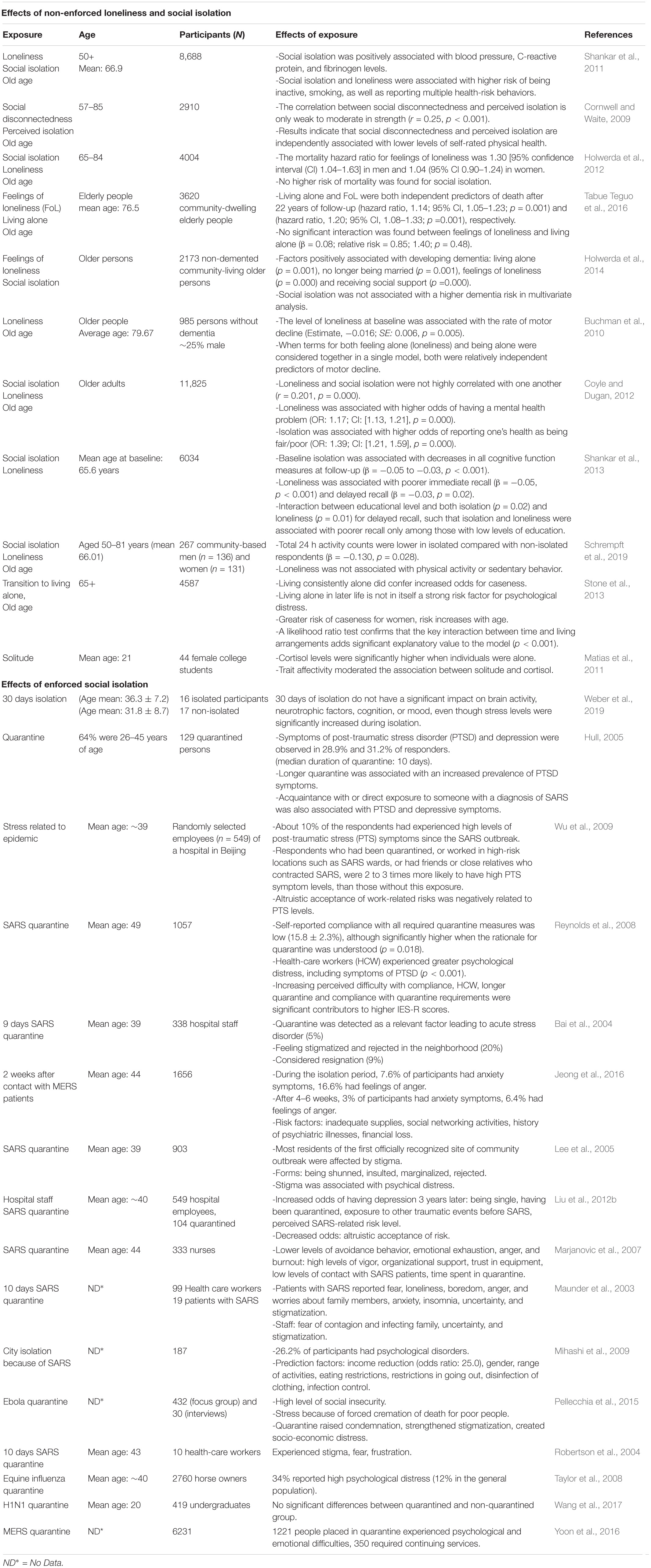
In humans, chronic social isolation can have detrimental health effects (House et al., 1988) (summarized in Table 1). Social isolation is associated with increased blood pressure, C-reactive protein, and fibrinogen levels (Shankar et al., 2011). It is also associated with an increased risk to be inactive (Shankar et al., 2011; Schrempft et al., 2019), have motor decline (Buchman et al., 2010) and impaired cognitive functions (Shankar et al., 2013). Loneliness or living alone has been linked with poorer immediate and delayed recall (Shankar et al., 2013) and dementia (Holwerda et al., 2014), as well as higher odds of mental health problems (Coyle and Dugan, 2012). Social isolation can as well result in health-risk behaviors, smoking (Shankar et al., 2011), and reduced self-related physical health (Cornwell and Waite, 2009; Coyle and Dugan, 2012). Therefore, social isolation affects physiology, cognition, and behavior in humans.
Effects of Social Isolation in Animal Models
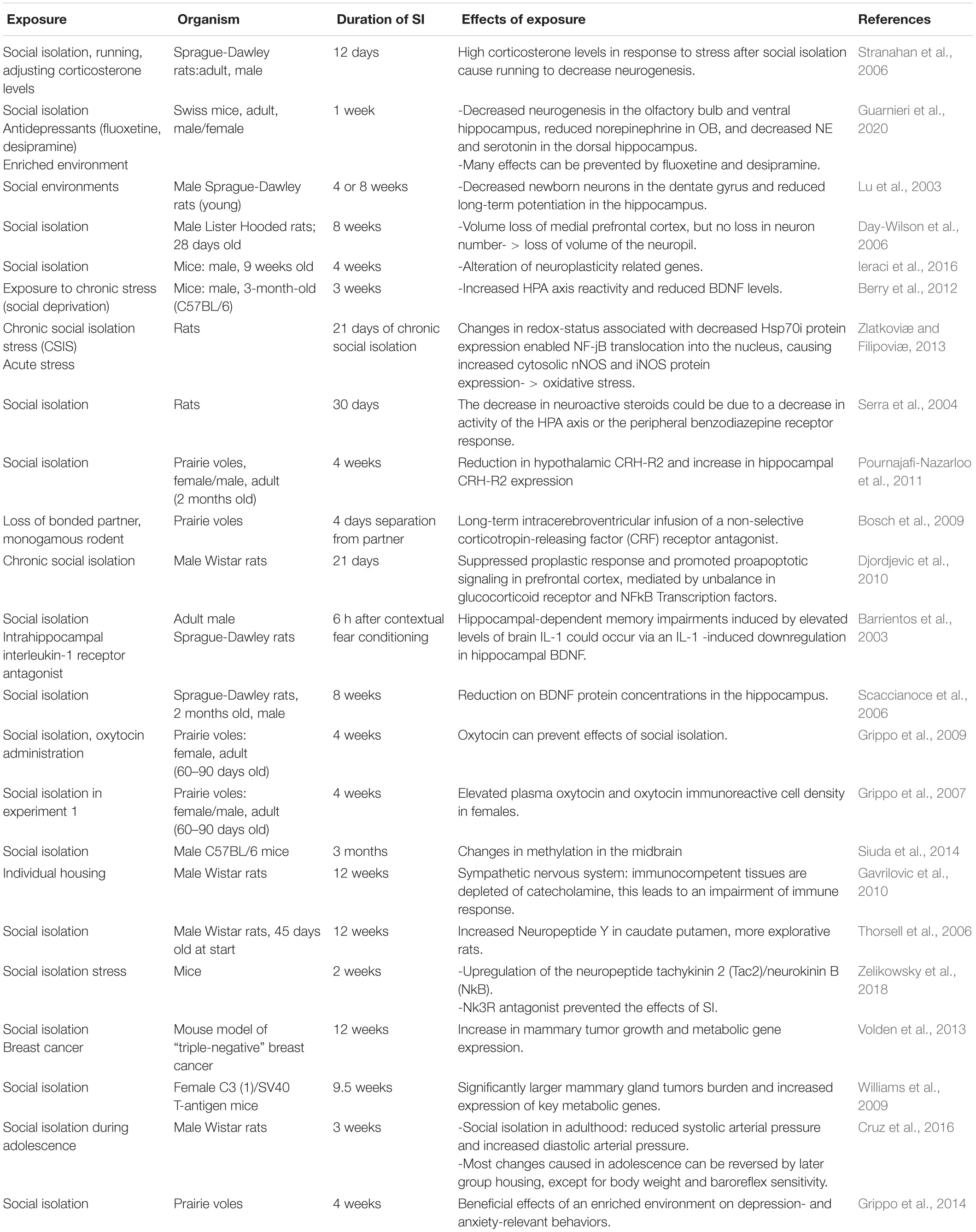
In rodents, social isolation has multiple effects on physiology and behavior (summarized in Table 2). Chronic social isolation (at least 2 weeks) results in complex behavioral responses characterized by increased aggressive behavior toward a submissive intruder, enhanced reactivity to footshock, and freezing to threatening ultrasonic stimulus (Zelikowsky et al., 2018). It also reduces time spent in the center of the arena during open field test (OFT) and increases the propensity to jump off an elevated plus maze (EPM) test (Zelikowsky et al., 2018). Chronically-isolated rodents spend less time interacting with a novel individual, but more time closer to a predator (Zelikowsky et al., 2018). They also have higher anxiety, depression, and anhedonia-like behaviors (Wallace et al., 2009), indicating that chronic social isolation alters behavioral responses in multiple ways.
Prolonged social isolation also affects different aspects of physiology. It can impair neurogenesis in the olfactory bulb (OB), the ventral hippocampus (VH), and the dentate gyrus (DG), and lead to reduced volume of some of these structures and the prefrontal cortex (Lu et al., 2003; Day-Wilson et al., 2006; Guarnieri et al., 2020). The loss of medial prefrontal cortex volume, but not its total number of neurons, resembles that observed in individuals with schizophrenia (Day-Wilson et al., 2006). Social isolation also affects the activity of the hypothalamic-pituitary-adrenal (HPA) axis, which controls the reaction to stress. In prairie voles, chronic isolation differentially affects the expression of the corticotropin-releasing factor receptor 2 (CRF2) between the hippocampus and the hypothalamus (Pournajafi-Nazarloo et al., 2011), two brain regions with major roles in regulating stress responses.
Notably, social isolation can promote tumor progression in animal models (Williams et al., 2009; Volden et al., 2013), and correlats with increased expression of key metabolic genes, upregulated lipid synthesis, and glucose metabolism in pre-malignant mammary gland (Williams et al., 2009) and mammary adipocytes (Volden et al., 2013).
Molecular Underpinnings of Social Isolation
Social Isolation and Loneliness Can Be Influenced by Genetic Variation
Loneliness is a social state strongly associated with mortality that is influenced by genetic variation (Gao et al., 2017; Day et al., 2018). A genome-wide association study (GWAS) including almost half a million participants from the UK Biobank study revealed the existence of genetic variants associated with loneliness and regular participation in social activities (Day et al., 2018). A total of 15 genomic loci were significantly associated with loneliness. Interestingly, the association was stronger in regions close to genes expressed preferentially in the brain where they are enriched for epigenetic modifications (Day et al., 2018), suggesting that loneliness can be influenced by genetic variants affecting the activity of regulatory elements in the brain. Interestingly, the expression of 8 genes was linked to susceptibility to loneliness: GPX1, C1QTNF4, C17orf58, MTCH2, BPTF, RP11-159N11.4, CRHR1-IT1, and PLEKHM1. The case of BPTF is of interest as it encodes the Bromodomain PHD finger transcription factor (BPTF) which is the largest subunit of the nucleosome remodeling factor (NURF), a major regulator of chromatin structure and gene expression (Barak et al., 2003; Stankiewicz et al., 2017). BPTF is highly expressed in the fetal brain and the brain of patients with neurodegenerative conditions, such as Alzheimer’s disease (Bowser et al., 1995). Mutations in BPTF have been found in patients with intellectual disability, speech delay, and microcephaly, while genetic inactivation of BPTF in Zebrafish leads to neurodevelopmental phenotypes (Stankiewicz et al., 2017). Therefore, genetic variation affecting the expression of BPTF could influence neurodevelopment and social states such as loneliness. Overall, results derived from GWAS suggest that in addition to life experiences, a specific genetic composition could influence social isolation and social interaction. However, whether these genetic associations truly influence brain development and function remains to be determined. Modeling genetic variants identified in humans using murine models and CRISPR-Cas9 editing (Zhu et al., 2019; Sandoval et al., 2020) could prove valuable to decipher the functionality of genetic variants associated with loneliness. Furthermore, it would be of great interest to increase the population diversity of GWAS to provide a comprehensive catalog of genetic variations influencing social behavior across human populations.
Social Isolation Induces Changes in Gene Expression
Conversely to the observation that loneliness is influenced by genetic makeup, social experiences can themselves alter gene transcription and have consequences for behavioral responses. In particular, social isolation can modulate gene expression across tissues in many species, from Drosophila to mammals (Wallace et al., 2009; Zelikowsky et al., 2018; Agrawal et al., 2020). In Drosophila, adult male flies exposed to social isolation for 4 days show robust changes in the expression of 90 genes mostly related to immune response (Agrawal et al., 2020). This is consistent with findings that social isolation modulates immune responses and induces inflammation (Powell et al., 2013; Cole et al., 2015), a condition also associated with depressive-like behaviors in animal models and depression in humans (Ma et al., 2020). The brain-specific neuropeptide Drosulfakinin (Dsk) was shown to be upregulated in the head of socially isolated males. It was proposed to act as a brake for aggressiveness induced by social isolation as Dsk knockdown increases aggressive behaviors of isolated male flies (Agrawal et al., 2020). Notably, its mammalian homolog cholecystokinin (CCK) regulates aggression and anxiety and has been implicated in panic disorder (Zwanzger et al., 2012; Katsouni et al., 2013). CCK transcription can also be modulated by other stressors such as maternal separation (Weidner et al., 2019).
In rodents, chronic social isolation stress can trigger widespread changes in the transcription of protein-coding and non-coding genes (Karelina et al., 2009; Wallace et al., 2009; Liu et al., 2012a; Jin et al., 2016; Kumari et al., 2016; Verma et al., 2016, 2018; Zelikowsky et al., 2018; Mavrikaki et al., 2019; Chang et al., 2020). In adult mice, social isolation for 8 weeks induces transcriptional changes in the myelin genes Mbop and Mobp in oligodendrocytes of the prefrontal cortex (PFC) (Liu et al., 2012a). Two weeks of social isolation induces a gradual transcription of Tact2 gene in the brain and peripheral endocrine tissues such as testis (Zelikowsky et al., 2018). Tact2 codes for the neuropeptide neurokinin B (NkB), necessary for behavioral responses observed in mice subjected to chronic social isolation (Zelikowsky et al., 2018). In rats, prolonged social isolation for 6–12 weeks induces changes in gene expression in the cortex and the nucleus accumbens shell (NAcSh), a brain region important for responses to emotional stimuli (Wallace et al., 2009; Kumari et al., 2016). In cortex, post-weaning social isolation increases the expression of the brain-derived neurotrophic factor (BDNF), the cAMP response element binding protein (CREB-1), and the histone acetyltransferase CREB-1 binding protein (CBP), but reduces the transcription of the histone deacetylase-2 (HDAC2). In the NAcSH, adult chronic social isolation also upregulates many genes coding for K+ channels and major regulatory proteins such as the activating transcription factor-2 (ATF2), Janus kinase and genes coding for epigenetic factors such as the histone deacetylase-4 (HDAC4) (Wallace et al., 2009). This suggests that chronic social isolation can potentially rewire gene regulatory networks by altering the amount of activity-dependent transcription factors and chromatin-modifying proteins.
Social isolation in rodents can also affect the expression of non-coding RNAs like miRNAs (Kumari et al., 2016; Verma et al., 2018; Mavrikaki et al., 2019; Antony et al., 2020, p. 181; Chang et al., 2020). Prolonged isolation of postnatal rats resulted in differential miRNAs expression in the anterodorsal bed nucleus of the stria terminalis (adBNS), a region involved in anxiety responses (Mavrikaki et al., 2019). A total of 12 miRNAs were differentially regulated in both socially-isolated males and females, with the majority being downregulated, e.g., miR-181c, miR-143, miR-29a, miR-434, and miR-22 (Mavrikaki et al., 2019). Interestingly, the level of miR-29a was also altered in other tissues such as the oral cavity (Yang et al., 2013), suggesting systemic responses to social isolation. miR-181c expression was also downregulated in the brain of isolated mice after stroke (Verma et al., 2018; Antony et al., 2020) while the levels of miR-181a are affected in blood of adult humans with a history of childhood trauma (Mavrikaki et al., 2019), suggesting that miR-181 family members could be a common target of stress responses in mammals.
Changes in miRNAs expression after social isolation can vary depending on sex (Kumari et al., 2016; Mavrikaki et al., 2019). For example, chronic social isolation upregulates miR-132, a direct target of CREB-1, and downregulates miR-134 in the cortex of female rats (Kumari et al., 2016). In female adBNS, twice more miRNAs were affected than in their male counterparts (Mavrikaki et al., 2019), and this correlated with an anxiety behavior (Kumari et al., 2016; Mavrikaki et al., 2019). These findings suggest that chronic social isolation can differentially modulate behavior and transcriptional programs depending on sex. While in females, target genes of miRNAs altered by social isolation are involved in drug addiction and MAPK signaling suggesting effects on reward pathways, in males, target genes are involved in GABAergic synapses thus affect inhibitory neurons (Mavrikaki et al., 2019). Consistently, social isolation increases the propensity to self-administer drugs and to develop addictive behaviors (Green et al., 2010). Overall, different lines of research strongly support that social isolation can alter transcriptional programs in the brain affecting both protein-coding and non-coding genes.
Transcription Factors and Epigenetic Mechanisms Modulate Behavioral Responses to Social Isolation
The mechanisms linking social isolation with changes in gene expression likely involve different molecular cascades with one of the major consequences being perturbed activity of transcription factors (Wallace et al., 2009; Kumari et al., 2016). In the rodent brain, the activity of the transcription factor CREB is diminished in NAcSh of rats exposed to chronic social isolation (Wallace et al., 2009). CREB has been associated with differential expression of a subset of genes, like those coding for K + channels, in the NAcSh of socially isolated rats. Notably, CREB overexpression is sufficient to revert the anxiety-like behavior observed in isolated individuals but not the anhedonia-like phenotype (Wallace et al., 2009). This suggests that CREB is a major player in the regulation of emotional hyper-reactivity in NAcSH and that additional molecular pathways likely regulate other behavioral abnormalities observed in socially-isolated animals. A major question regarding the role of CREB in social isolation is the molecular nature of its reduced activity during prolonged social isolation. To date, it is unknown whether transcriptional or post-transcriptional mechanisms operating in the NAcSh are responsible for its reduced regulatory activity during chronic social isolation.
Classical epigenetic mechanisms for the control of gene expression are also implicated in the effects of prolonged social isolation (Weaver et al., 2004; Murgatroyd et al., 2009; Gapp et al., 2014; Siuda et al., 2014; Wang et al., 2017). Intermittent social isolation in early postnatal life in rodents, such as induced by maternal separation, can modulate DNA methylation and histone post-translational modifications at regulatory elements of genes involved in stress reactivity including the glucocorticoid receptor (GR) gene (Nr3c1) (Weaver et al., 2004) and the mineralocorticoid receptor (MR) gene (Nr3c2) (Gapp et al., 2014). This has been associated with a rewiring of stress responses and behavioral adaptation. Chronic social isolation during the juvenile period can also alter the epigenome. Pups at postnatal day (PND) 21 subjected to social isolation for 2 months show a global increase in the level of the repressive histone post-translational modification H3K9me2 in neurons, an effect correlated with increased transcription of the H3K9me2 histone methyltransferase (HMT) G9a and GLP in the hippocampus (Wang et al., 2017). In adult male mice, chronic social isolation for 3 months induced a significant global increase in DNA methylation, H3K4 di, and trimethylation as well as a trend toward an increase in the global levels for H3K9ac, in the midbrain (Siuda et al., 2014). In all cases, the increase in epigenetic modifications was associated with an increase in the catalytic processes leading to such epigenetic modifications. For example, H3K4 HMT activity was significantly enhanced in the midbrain of socially isolated male mice, which could suggest increased transcription of genes coding for H3K4 HMT (Siuda et al., 2014). Prolonged social isolation also increased the transcription of genes coding for HDACs such as Hdac1 and Hdac3 which correlated with decreased CpG methylation at their promoter regions (Siuda et al., 2014), supporting the hypothesis that chronic isolation can perturb gene regulatory networks by altering epigenetic modifiers. In contrast, the transcription of the gene coding for the serotonin transporter Slc6a4 was markedly reduced by social isolation and this correlated with increased DNA methylation at its promoter region (Siuda et al., 2014).
Non-coding RNAs Are Major Regulators of Social Behavior
Non-coding RNAs such as miRNAs and lncRNAs are major regulators of gene expression across the animal kingdom (Jonas and Izaurralde, 2015; Engreitz et al., 2016; Kim et al., 2016; Li and Fu, 2019). Although different lines of evidence suggest that ncRNAs are transcriptionally altered in the brain of rodents after social isolation, direct and functional evidence on their contribution to behavioral and physiological consequences of prolonged social isolation is still sparse (Verma et al., 2018; Antony et al., 2020; Chang et al., 2020). However miRNAs and lncRNAs were proven to modulate social behaviors which are also altered as the result of prolonged social isolation (Haramati et al., 2011; Dias et al., 2014; Issler et al., 2014; Jin et al., 2016; Zhu et al., 2017; Cheng et al., 2018; Lackinger et al., 2019; Labonté et al., 2020; Ma et al., 2020).
MiRNAs
Different miRNAs have been documented to modulate aggressive-, anxiety-, and depression-like behaviors as responses to prolonged social isolation. For example, miR-206 is responsible for the stress-induced aggressive behavior of socially isolated mice via direct targeting of BDNF mRNA in the ventral hippocampus (Chang et al., 2020). MiR-34c is downregulated in the brain of socially isolated female rats (Mavrikaki et al., 2019) and it is responsive to chronic stress in the adult central nucleus of the amygdala (CeA) where it has been shown to have an anxiolytic effect when overexpressed (Haramati et al., 2011). Since prolonged social isolation is a form of chronic stress and anxiety a behavioral response of socially isolated female rodents (Kumari et al., 2016; Mavrikaki et al., 2019), miR-34c could be a modulator of anxiolytic responses due to prolonged social isolation.
MiR-135 can modulate serotonin functions by targeting the serotonin transporter Slc6a4 (Issler et al., 2014), which is downregulated in the midbrain of socially isolated adult mice (Siuda et al., 2014). Consistently, deletion of miR-135 gene in serotonergic neurons results in anxiety- and depression-like behaviors while miR-135 overexpression induces resilience to the behavioral effects of chronic social stress (Issler et al., 2014). The miRNA cluster miR-17-92 is of particular interest as it can also regulate anxiety- and depression-like behaviors by targeting transcripts of the glucocorticoid receptor (GR) pathway in the adult brain (Jin et al., 2016). Deletion of the miRNA cluster in neural progenitors in the adult brain resulted in mice displaying anxiety-, depression-, and anhedonia-like behaviors while miR-17-92 cluster overexpression had anxiolytic and antidepressant-like effects (Jin et al., 2016). Notably, anxiety-, depression-, and anhedonia-like behaviors are all behavioral manifestations of adult rodents exposed to chronic social isolation (Wallace et al., 2009; Zelikowsky et al., 2018) which suggest that chronic pervasive stress can modulate the expression of miRNAs and in such way, impact behavior. In support of this, chronic stress results in reduced expression of the miR-17-92 cluster while the overexpression of the miR-17-92 cluster was anxiolytic and protected against the deleterious effect of chronic stress on neurogenesis (Jin et al., 2016). MiR-137 is another important modulator of social behavior. Heterozygous mice for miR-137 show impaired social behaviors, such as reduced social preference toward other mice, as well as impaired response to social novelty (Cheng et al., 2018), all behavioral manifestations of prolonged social isolation in rodents.
The miR-379-410 cluster is the best-characterized group of miRNAs with a demonstrated role in fine-tuning social behavior in mammals (Lackinger et al., 2019). It is specifically expanded in placental animals and contains 38 miRNAs with documented roles in neuronal processes. Constitutive removal of the entire cluster results in hyper-social behavior characterized by increased ultrasonic vocalizations both during early and juvenile postnatal life, exaggerated reciprocal social interactions, and increased social approach behavior, suggesting that such miRNAs as a group can function to buffer social behavior in mammals (Lackinger et al., 2019). Knockout mice also had reduced repetitive behaviors and attenuated anxiety-related behaviors. Molecularly, the loss of the miR-379-410 cluster leads to a major up-regulation of the transcript levels for more than 3,000 genes in neurons, consistent with the role of miRNAs in suppressing gene expression. Interestingly, some of the up-regulated genes code for glutamate receptor components which was linked with increased neuronal excitability in the hippocampus and hyper-social behavior (Lackinger et al., 2019). Therefore, the miR-379-410 cluster is a genomic regulatory hub for the fine-tuning of social behavior in mammals. Whether members of the cluster are implicated in behavioral abnormalities due to prolonged social isolation remains to be determined.
While it is clear that miRNAs are transcriptionally dysregulated by social isolation and some of them directly modulate behaviors characteristic of chronically isolated animals, manipulating specific miRNAs in vivo has recently emerged as a promising therapeutic approach to ameliorate the negative effects of social isolation on behavior and physiology (Verma et al., 2018; Antony et al., 2020; Chang et al., 2020). For example, inhibition of miR-206 in the hippocampus of socially isolated mice or intranasal administration of an antagonist of miR-206 eliminates stress-provoked attacks via BDNF upregulation (Chang et al., 2020). Also, social isolation can negatively influence stroke recovery in humans and rodents and this has been associated with the dysregulation of miRNAs, such as miR-181c and miR-141, in a mouse model of stroke (Verma et al., 2018; Antony et al., 2020). Mice that were socially isolated post-stroke showed a gradual decrease in the levels of miR-181c in the ipsilateral cortex as compared with group-housed mice also subjected to stroke. Remarkably, the systemic upregulation of miR-181c using a miRNA mimic significantly increased miR-181c levels in the brain and improved survival rate after stroke in isolated mice. This also partially rescued locomotor effects and ameliorated anxiety. Molecularly, the re-establishment of miR-181c levels reduced glial activation in isolated mice (Antony et al., 2020), a remarkable finding as glia activation after stroke has been related to increased inflammation and poorer prognosis (Xu et al., 2020). This data suggest that social isolation could compromise neuroinflammatory responses in the brain after stroke. In support of this, social isolation after stroke impairs the transcriptional upregulation of interleukin-6 (IL-6) in the brain (Karelina et al., 2009). While IL-6 is a cytokine involved in the induction of inflammatory responses, IL-6 induction in the brain is neuroprotective (Loddick et al., 1998). Importantly, the systemic inhibition of miR-141c, which is upregulated in the brain of socially isolated mice after stroke, resulted in the transcriptional upregulation of IL-6 (Verma et al., 2018). Thus, miRNAs act as major modulators of inflammatory responses via regulation of pro-inflammatory genes in the context of social isolation after stroke.
LncRNAs
LncRNAs can also affect social behavior in mice through different mechanisms. The antisense lncRNA of synapsin II (AtLAS) is differentially expressed in the mPFC between dominant and subordinate mice (Ma et al., 2020) and its downregulation in excitatory neurons of the mPFC is sufficient to establish social dominance in grouped mice. Since chronically isolated mice have altered behaviors toward other individuals, such as increased aggression but also blunted response to social novelty (Zelikowsky et al., 2018), it is possible that lncRNAs are important modulators of behavioral responses due to chronic social isolation in mammals. Consistently, aggressive behaviors have also been associated with changes in lncRNAs expression (Punzi et al., 2019; Labonté et al., 2020). The monoamine oxidase A (MAOA) associated ncRNA (MAALIN) is a lncRNA located in the 3′ intergenic region separating the tail-to-tail oriented MAO genes A and B. In humans, the promoter of MAALIN is hypomethylated in neurons of the DG from suicidal subjects with a history of impulsive aggressive disorders and this correlates with lower expression of MAOA gene, which has been implicated in aggressive disorders both in humans and animals (Labonté et al., 2020). Overexpression of MAALIN in the hippocampus of aggressive mice induces a discrete downregulation of MAOA and results in a trend for increased duration of attacks toward other mice, suggesting that MAALIN could affect aggressive behavior (Labonté et al., 2020), a stereotypic behavioral response for male mice exposed to chronic social isolation.
Neuropeptides Are Major Drivers of Behavioral Responses to Social Isolation
Neuropeptides are major modulators of the behavioral effects observed during extended periods of social isolation across the animal kingdom. In Drosophila, the neuropeptides drosulfakinin and tachykinin modulate aggressive behavior in isolated male flies (Asahina et al., 2014; Agrawal et al., 2020). In mice, the expression of the neuropeptide NkB is sufficient and necessary for the behavioral abnormalities observed in socially isolated mice (Zelikowsky et al., 2018). Interestingly, NkB acts regionally in different brain areas to modulate specific behavioral responses due to chronic social isolation stress (Zelikowsky et al., 2018). Therefore, neuropeptides in conjunction with activity-dependent transcription factors, epigenetic modifiers, and ncRNAs are major modulators of behavioral and physiological responses to social isolation.
Behavioral Implications of Quarantine During Epidemics: A Cautionary Tale
In the past centuries, the timely implementation of isolation and quarantine of human populations has shown to be an effective public health intervention to stop the spread of viruses, such as the Ebola virus, MERS-CoV, SARS-Cov, and more recently, SARS-CoV2, the causal agent of COVID-19 (Hull, 2005; Pellecchia et al., 2015; Yoon et al., 2016; Prem et al., 2020). Countries all over the world have applied this strategy, resulting in mandatory or voluntary confinement for several months for more than a third of the world’s population up to this point.
Various countries pursued different approaches to prevent and reduce the spread of the virus. The first and strictest type of quarantine was enforced in Wuhan, China, the origin of the coronavirus outbreak (Prem et al., 2020). In some areas of the city, residents were completely forbidden to leave their home. Authorities went from door to door for health checks and forced the ill into isolation (Wuhan’s coronavirus outbreak: life inside the quarantine). Italy was the second country to enforce quarantine, then most European countries followed with different level of restrictions. Some countries pursued a more relaxed approach, such as Sweden where confinement was not mandatory, resulting in differences on the overall infectious and death rate due to COVID-19 (Habib, 2020).
While isolation refers to the separation of infected people from those who are healthy, quarantine separates and restricts the movement of people who might be infected but are not yet symptomatic. Physical distancing reduces the frequency and closeness of social contact between people. Although quarantine has been successful in slowing down the spread of the virus, poor implementation can cause additional problems in the exposed people (Pellecchia et al., 2015; Brooks et al., 2020; Buttell and Ferreira, 2020). The current quarantine due to COVID-19 has increased domestic violence, fear of people losing their jobs, reduced physical activity, altered sleep, and increased anxiety (KANTAR, 2020; How the Pandemic Could Be Messing With Your Sleep; Agren et al., 2020; Bouillon-Minois et al., 2020; Economic Commission for Latin America and the Caribbean, 2020; Mahase, 2020; Mazza et al., 2020; Spinelli et al., 2020; Thomas et al., 2020). These effects can even be more pronounced in people in developing countries where most of the population lives under the poverty line, including nations in Africa, Asia, and Latin America. Residents of such countries are in a tremendous hazard on suffering from lasting effects of forced confinement as they can not fulfill even their most basic need. They experience the quarantine as a major physical and psychological stressor for extended periods of time (Madhav et al., 2017; Yatham et al., 2018; Agren et al., 2020; Economic Commission for Latin America and the Caribbean, 2020).
More than 50 years of research in animal models and humans have conclusively shown the detrimental effects of chronic stress on health, highlighting the necessity for more empathic interventions to protect or reduce the sequelae of confinement on mental health and well-being of the population. Simple yet effective strategies could be implemented to reduce social isolation and perceived loneliness among older people, which are a sector of the population at risk to experience the detrimental effects of social isolation (Gardiner et al., 2018). Animal interventions, like animal-assisted therapy or having a pet has been shown to alleviate loneliness in the elderly (Shankar et al., 2011; Krause-Parello, 2012). The use of electronic devices, specifically computer and internet in older adults has also been found to decrease loneliness (Heo et al., 2015). In this regard, the use of a mobile phone for sociability has been associated with decreased loneliness, particularly when used in the context of face-to-face interactions (Wang et al., 2018). Therefore, the knowledge gained by previous research on the biological effects of social isolation on behavior has been an important driving factor for the realization that quarantine can have long-lasting effects on the population.
Conclusion
Prolonged social isolation has detrimental effects on humans and animals. In humans, chronic social isolation perturbs physical and mental health and we are just starting to uncover the molecular mechanisms driving behavioral effects associated with social withdrawal. Evidence derived from different animal models strongly suggests that social isolation can induce transcriptional changes in different brain areas fundamental for memory and cognition and also relevant for the modulation of mood and even addictive behaviors. Some of the affected genes are major transcriptional regulators such as the AP-1 transcription factors and CREB, both mediators of transcriptional responses due to neuronal activation in mammals (Yap and Greenberg, 2018). Furthermore, important epigenetic modifiers such as the H3K9me2 histone methyltransferase G9a and histone deacetylases like HDAC-2 and -4, as well as regulatory ncRNAs like miRNAs are also dysregulated, suggesting that social isolation could remodel chromatin and impact steady-state or stimulus-dependent transcriptional responses. While current findings suggest such a possibility, direct causal evidence linking the potential mediators, e.g., transcription factors and epigenetic modulators, with the establishment and maintenance of behavioral and physiological abnormalities associated with social isolation, is still sparse. A major missing information also is the identification of signaling pathways responsible for transcriptional events observed in the brain of socially isolated animals such as CREB activation or transcriptional downregulation of HDACs. Also, the molecular events leading to specific regulation of a subset of miRNAs that modulate important signaling molecules such as BNDF and IL-6 during social isolation are not known.
Furthermore, although available evidence suggests that GR signaling is implicated in the response to acute social isolation in mice (Kamal et al., 2014), whether it contributes to transcriptional effects observed during chronic social isolation is unknown. Based on the available evidence, we envision that chronic social isolation induces remodeling of chromatin structure and organization as a consequence of exposure to chronic stress. Such modification could affect not just brain cells but also other tissues, persistently modifying regulatory programs which in turn change behavior and physiology.
From a public health perspective, major attention should be paid to the physiological and psychological consequences of social withdrawal on the general population. Given that loneliness in humans has been documented to be linked to all-cause increased mortality and with an effect on mortality comparable to smoking, it is fundamental to gain better knowledge of the molecular mechanisms that promote the behavioral and physiological effects of isolation with the long-term goal to develop new pharmacological and non-pharmacological interventions. While in most cases, social isolation has detrimental effects on the exposed individual in humans and animals, it is possible that some individuals show some resilience. It may be linked to better coping strategies, a isolation habituated state due to a lifestyle based on loneliness, or a natural lower sensitivity to such social stress.
Finally, current actions to mitigate the pandemic of COVID-19 is a call to revisit and implement the best possible public health interventions to protect people against infectious diseases without affecting their physical and mental health. The imposed regulations by governments around the world may have consequences that people do not anticipate and may reverberate for years and possibly decades. Given that the emergence and spread of viruses that infect humans are and will be a constant thread for humankind, a more thoughtful strategy is needed to reduce social interaction while taking into consideration the extraordinary impact that social interactions can have in life.
Author Contributions
AJ, ZL, and VS wrote a draft of the review, and RGA-M and IMM finished it.
Funding
The Mansuy lab was primarily funded by the University Zurich, the ETH Zurich, the Swiss National Science Foundation (31003A_175742/1), and EU grant EarlyCause (project Nr. 848158). RGA-M was funded by an ETHZ postdoctoral fellowship (20-1 FEL-28).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agrawal, P., Kao, D., Chung, P., and Looger, L. L. (2020). The neuropeptide drosulfakinin regulates social isolation-induced aggression in Drosophila. J. Exp. Biol. 223:jeb207407. doi: 10.1242/jeb.207407
Agren, D., Goñi, U., Phillips, T., and Daniels, J. P. (2020). Lockdowns Leave Poor Latin Americans with Impossible Choice: Stay Home or Feed Families. London: The Guardian.
Antony, M., Scranton, V., Srivastava, P., and Verma, R. (2020). Micro RNA 181c-5p: a promising target for post-stroke recovery in socially isolated mice. Neurosci. Lett. 715:134610. doi: 10.1016/j.neulet.2019.134610
Asahina, K., Watanabe, K., Duistermars, B. J., Hoopfer, E., González, C. R., Eyjólfsdóttir, E. A., et al. (2014). Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell 156, 221–235. doi: 10.1016/j.cell.2013.11.045
Bai, Y., Lin, C.-C., Lin, C.-Y., Chen, J.-Y., Chue, C.-M., and Chou, P. (2004). Survey of stress reactions among health care workers involved with the SARS outbreak. Psychiatr. Serv. 55, 1055–1057. doi: 10.1176/appi.ps.55.9.1055
Barak, O., Lazzaro, M. A., Lane, W. S., Speicher, D. W., Picketts, D. J., and Shiekhattar, R. (2003). Isolation of human NURF: a regulator of engrailed gene expression. EMBO J. 22, 6089–6100. doi: 10.1093/emboj/cdg582
Barrientos, R. M., Sprunger, D. B., Campeau, S., Higgins, E. A., Watkins, L. R., Rudy, J. W., et al. (2003). Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience 121, 847–853. doi: 10.1016/s0306-4522(03)00564-565
Berry, A., Bellisario, V., Capoccia, S., Tirassa, P., Calza, A., Alleva, E., et al. (2012). Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology 37, 762–772. doi: 10.1016/j.psyneuen.2011.09.007
Bludau, A., Royer, M., Meister, G., Neumann, I. D., and Menon, R. (2019). Epigenetic regulation of the social brain. Trends Neurosci. 42, 471–484. doi: 10.1016/j.tins.2019.04.001
Bosch, O. J., Nair, H. P., Ahern, T. H., Neumann, I. D., and Young, L. J. (2009). The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology 34, 1406–1415. doi: 10.1038/npp.2008.154
Bouillon-Minois, J.-B., Clinchamps, M., and Dutheil, F. (2020). Coronavirus and quarantine: catalysts of domestic violence. Viol. Against Women doi: 10.1177/1077801220935194
Bowser, R., Giambrone, A., and Davies, P. (1995). FAC1, a novel gene identified with the monoclonal antibody Alz50, is developmentally regulated in human brain. Dev. Neurosci. 17, 20–37. doi: 10.1159/000111270
Brooks, S. K., Webster, R. K., Smith, L. E., Woodland, L., Wessely, S., Greenberg, N., et al. (2020). The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 395, 912–920. doi: 10.1016/S0140-6736(20)30460-30468
Buchman, A. S., Boyle, P. A., Wilson, R. S., James, B. D., Leurgans, S. E., Arnold, S. E., et al. (2010). Loneliness and the rate of motor decline in old age: the rush memory and aging project, a community-based cohort study. BMC Geriatr. 10:77. doi: 10.1186/1471-2318-10-77
Buttell, F., and Ferreira, R. J. (2020). The hidden disaster of COVID-19: intimate partner violence. Psychol. Trauma 12, S197–S198. doi: 10.1037/tra0000646
Chang, C.-H., Kuek, E. J. W., Su, C.-L., and Gean, P.-W. (2020). MicroRNA-206 regulates stress-provoked aggressive behaviors in post-weaning social isolation mice. Mol. Ther. Nucleic Acids 20, 812–822. doi: 10.1016/j.omtn.2020.05.001
Chen, P., and Hong, W. (2018). Neural circuit mechanisms of social behavior. Neuron 98, 16–30. doi: 10.1016/j.neuron.2018.02.026
Cheng, Y., Wang, Z.-M., Tan, W., Wang, X., Li, Y., Bai, B., et al. (2018). Partial loss of psychiatric risk gene Mir137 in mice causes repetitive behavior and impairs sociability and learning via increased Pde10a. Nat. Neurosci. 21, 1689–1703. doi: 10.1038/s41593-018-0261-267
Cole, S. W., Capitanio, J. P., Chun, K., Arevalo, J. M. G., Ma, J., and Cacioppo, J. T. (2015). Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. PNAS 112, 15142–15147. doi: 10.1073/pnas.1514249112
Cole, S. W., Hawkley, L. C., Arevalo, J. M., Sung, C. Y., Rose, R. M., and Cacioppo, J. T. (2007). Social regulation of gene expression in human leukocytes. Genome Biol. 8:R189. doi: 10.1186/gb-2007-8-9-r189
Cornwell, E. Y., and Waite, L. J. (2009). Social disconnectedness, perceived isolation, and health among older adults. J. Health Soc. Behav. 50, 31–48. doi: 10.1177/002214650905000103
Coyle, C. E., and Dugan, E. (2012). Social isolation, loneliness and health among older adults. J. Aging Health 24, 1346–1363. doi: 10.1177/0898264312460275
Cruz, F. C., Duarte, J. O., Leão, R. M., Hummel, L. F. V., Planeta, C. S., and Crestani, C. C. (2016). Adolescent vulnerability to cardiovascular consequences of chronic social stress: immediate and long-term effects of social isolation during adolescence. Dev. Neurobiol. 76, 34–46. doi: 10.1002/dneu.22297
Day, F. R., Ong, K. K., and Perry, J. R. B. (2018). Elucidating the genetic basis of social interaction and isolation. Nat. Commun. 9:2457. doi: 10.1038/s41467-018-04930-4931
Day-Wilson, K. M., Jones, D. N. C., Southam, E., Cilia, J., and Totterdell, S. (2006). Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience 141, 1113–1121. doi: 10.1016/j.neuroscience.2006.04.048
Dias, B. G., Goodman, J. V., Ahluwalia, R., Easton, A. E., Andero, R., and Ressler, K. J. (2014). Amygdala-dependent fear memory consolidation via miR-34a and notch signaling. Neuron 83, 906–918. doi: 10.1016/j.neuron.2014.07.019
Djordjevic, A., Adzic, M., Djordjevic, J., and Radojcic, M. B. (2010). Chronic social isolation suppresses proplastic response and promotes proapoptotic signalling in prefrontal cortex of wistar rats. J. Neurosci. Res. 88, 2524–2533. doi: 10.1002/jnr.22403
Economic Commission for Latin America and the Caribbean (2020). Report on the Economic Impact of Coronavirus Disease (COVID-19) on Latin America and the Caribbean. Santiago: CEPAL.
Engreitz, J. M., Ollikainen, N., and Guttman, M. (2016). Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat. Rev. Mol. Cell Biol. 17, 756–770. doi: 10.1038/nrm.2016.126
Gao, J., Davis, L. K., Hart, A. B., Sanchez-Roige, S., Han, L., Cacioppo, J. T., et al. (2017). Genome-wide association study of loneliness demonstrates a role for common variation. Neuropsychopharmacology 42, 811–821. doi: 10.1038/npp.2016.197
Gapp, K., Soldado-Magraner, S., Alvarez-Sánchez, M., Bohacek, J., Vernaz, G., Shu, H., et al. (2014). Early life stress in fathers improves behavioural flexibility in their offspring. Nat. Commun. 5:5466. doi: 10.1038/ncomms6466
Gardiner, C., Geldenhuys, G., and Gott, M. (2018). Interventions to reduce social isolation and loneliness among older people: an integrative review. Health Soc. Care Commun. 26, 147–157. doi: 10.1111/hsc.12367
Gavrilovic, L., Spasojevic, N., and Dronjak, S. (2010). Chronic individual housing-induced stress decreased expression of catecholamine biosynthetic enzyme genes and proteins in spleen of adult rats. Neuroimmunomodulation 17, 265–269. doi: 10.1159/000290042
Green, T. A., Alibhai, I. N., Roybal, C. N., Winstanley, C. A., Theobald, D. E. H., Birnbaum, S. G., et al. (2010). Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol. Psychiatry 67, 28–35. doi: 10.1016/j.biopsych.2009.06.022
Grippo, A. J., Gerena, D., Huang, J., Kumar, N., Shah, M., Ughreja, R., et al. (2007). Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32, 966–980. doi: 10.1016/j.psyneuen.2007.07.004
Grippo, A. J., Ihm, E., Wardwell, J., McNeal, N., Scotti, M.-A. L., Moenk, D. A., et al. (2014). The effects of environmental enrichment on depressive and anxiety-relevant behaviors in socially isolated prairie voles. Psychosom. Med. 76, 277–284. doi: 10.1097/PSY.0000000000000052
Grippo, A. J., Trahanas, D. M., Zimmerman, R. R., Porges, S. W., and Carter, C. S. (2009). Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology 34, 1542–1553. doi: 10.1016/j.psyneuen.2009.05.017
Guarnieri, L. O., Pereira-Caixeta, A. R., Medeiros, D. C., Aquino, N. S. S., Szawka, R. E., Mendes, E. M. A. M., et al. (2020). Pro-neurogenic effect of fluoxetine in the olfactory bulb is concomitant to improvements in social memory and depressive-like behavior of socially isolated mice. Transl. Psychiatry 10, 1–14. doi: 10.1038/s41398-020-0701-705
Habib, H. (2020). Has Sweden’s controversial covid-19 strategy been successful? BMJ 369:m2376. doi: 10.1136/bmj.m2376
Haramati, S., Navon, I., Issler, O., Ezra-Nevo, G., Gil, S., Zwang, R., et al. (2011). MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J. Neurosci. 31, 14191–14203. doi: 10.1523/JNEUROSCI.1673-11.2011
Heo, J., Chun, S., Lee, S., Lee, K. H., and Kim, J. (2015). Internet use and well-being in older adults. Cyberpsychol. Behav. Soc. Netw. 18, 268–272. doi: 10.1089/cyber.2014.0549
Hilakivi, L. A., Ota, M., and Lister, R. G. (1989). Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral “despair.”. Pharmacol. Biochem. Behav. 33, 371–374. doi: 10.1016/0091-3057(89)90516-90519
Holwerda, T. J., Beekman, A. T. F., Deeg, D. J. H., Stek, M. L., van Tilburg, T. G., Visser, P. J., et al. (2012). Increased risk of mortality associated with social isolation in older men: only when feeling lonely? Results from the Amsterdam Study of the Elderly (AMSTEL). Psychol. Med. 42, 843–853. doi: 10.1017/S0033291711001772
Holwerda, T. J., Deeg, D. J. H., Beekman, A. T. F., van Tilburg, T. G., Stek, M. L., Jonker, C., et al. (2014). Feelings of loneliness, but not social isolation, predict dementia onset: results from the Amsterdam Study of the Elderly (AMSTEL). J. Neurol. Neurosurg. Psychiatry 85, 135–142. doi: 10.1136/jnnp-2012-302755
House, J. S., Landis, K. R., and Umberson, D. (1988). Social relationships and health. Science 241, 540–545. doi: 10.1126/science.3399889
Hull, H. F. (2005). SARS control and psychological effects of quarantine. TorontoCanada. Emerg. Infect. Dis. 11, 354–355. doi: 10.3201/eid1102.040760
Hwang, J.-Y., Aromolaran, K. A., and Zukin, R. S. (2017). The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat. Rev. Neurosci. 18, 347–361. doi: 10.1038/nrn.2017.46
Ieraci, A., Mallei, A., and Popoli, M. (2016). Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural. Plast. 2016:6212983. doi: 10.1155/2016/6212983
Issler, O., Haramati, S., Paul, E. D., Maeno, H., Navon, I., Zwang, R., et al. (2014). MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 83, 344–360. doi: 10.1016/j.neuron.2014.05.042
Jeong, H., Yim, H. W., Song, Y.-J., Ki, M., Min, J.-A., Cho, J., et al. (2016). Mental health status of people isolated due to middle east respiratory syndrome. Epidemiol. Health 38:e2016048. doi: 10.4178/epih.e2016048
Jin, J., Kim, S.-N., Liu, X., Zhang, H., Zhang, C., Seo, J.-S., et al. (2016). miR-17-92 Cluster regulates adult hippocampal neurogenesis, anxiety, and depression. Cell Rep. 16, 1653–1663. doi: 10.1016/j.celrep.2016.06.101
Jonas, S., and Izaurralde, E. (2015). Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 16, 421–433. doi: 10.1038/nrg3965
Kamal, A., Ramakers, G. M. J., Altinbilek, B., and Kas, M. J. H. (2014). Social isolation stress reduces hippocampal long-term potentiation: effect of animal strain and involvement of glucocorticoid receptors. Neuroscience 256, 262–270. doi: 10.1016/j.neuroscience.2013.10.016
Karelina, K., Norman, G. J., Zhang, N., Morris, J. S., Peng, H., and DeVries, A. C. (2009). Social isolation alters neuroinflammatory response to stroke. Proc. Natl. Acad. Sci. U.S.A. 106, 5895–5900. doi: 10.1073/pnas.0810737106
Katsouni, E., Zarros, A., Skandali, N., Tsakiris, S., and Lappas, D. (2013). The role of cholecystokinin in the induction of aggressive behavior: a focus on the available experimental data (review). Acta Physiol. Hung 100, 361–377. doi: 10.1556/APhysiol.100.2013.4.1
Kim, D., Sung, Y. M., Park, J., Kim, S., Kim, J., Park, J., et al. (2016). General rules for functional microRNA targeting. Nat. Genet. 48, 1517–1526. doi: 10.1038/ng.3694
Krause-Parello, C. A. (2012). Pet ownership and older women: the relationships among loneliness, pet attachment support, human social support, and depressed mood. Geriatr. Nurs. 33, 194–203. doi: 10.1016/j.gerinurse.2011.12.005
Kumari, A., Singh, P., Baghel, M. S., and Thakur, M. K. (2016). Social isolation mediated anxiety like behavior is associated with enhanced expression and regulation of BDNF in the female mouse brain. Physiol. Behav. 158, 34–42. doi: 10.1016/j.physbeh.2016.02.032
Labonté, B., Abdallah, K., Maussion, G., Yerko, V., Yang, J., Bittar, T., et al. (2020). Regulation of impulsive and aggressive behaviours by a novel lncRNA. Mol. Psychiatry doi: 10.1038/s41380-019-0637-634 Online ahead of print.
Lackinger, M., Sungur, Ö, Daswani, R., Soutschek, M., Bicker, S., Stemmler, L., et al. (2019). A placental mammal-specific microRNA cluster acts as a natural brake for sociability in mice. EMBO Rep. 20:e46429. doi: 10.15252/embr.201846429
Lee, S., Chan, L. Y. Y., Chau, A. M. Y., Kwok, K. P. S., and Kleinman, A. (2005). The experience of SARS-related stigma at Amoy Gardens. Soc. Sci. Med. 61, 2038–46. doi: 10.1016/j.socscimed.2005.04.010
Li, X., and Fu, X.-D. (2019). Chromatin-associated RNAs as facilitators of functional genomic interactions. Nat. Rev. Genet. 20, 503–519. doi: 10.1038/s41576-019-0135-131
Liu, J., Dietz, K., DeLoyht, J. M., Pedre, X., Kelkar, D., Kaur, J., et al. (2012a). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623. doi: 10.1038/nn.3263
Liu, X., Kakade, M., Fuller, C. J., Fan, B., Fang, Y., Kong, J., et al. (2012b). Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr. Psychiatry 53, 15–23. doi: 10.1016/j.comppsych.2011.02.003
Loddick, S. A., Turnbull, A. V., and Rothwell, N. J. (1998). Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 18, 176–179. doi: 10.1097/00004647-199802000-199802008
Lu, L., Bao, G., Chen, H., Xia, P., Fan, X., Zhang, J., et al. (2003). Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp. Neurol. 183, 600–609. doi: 10.1016/s0014-4886(03)00248-246
Ma, M., Xiong, W., Hu, F., Deng, M.-F., Huang, X., Chen, J.-G., et al. (2020). A novel pathway regulates social hierarchy via lncRNA AtLAS and postsynaptic synapsin IIb. Cell Res. 30, 105–118. doi: 10.1038/s41422-020-0273-271
Madhav, N., Oppenheim, B., Gallivan, M., Mulembakani, P., Rubin, E., and Wolfe, N. (2017). ““Pandemics: risks, impacts, and mitigation,”,” in Disease Control Priorities: Improving Health and Reducing Poverty, eds D. T. Jamison, H. Gelband, S. Horton, P. Jha, R. Laxminarayan, C. N. Mock, et al. (Washington (DC: The International Bank for Reconstruction and Development / The World Bank).
Mahase, E. (2020). Covid-19: EU states report 60% rise in emergency calls about domestic violence. BMJ 2020:369. doi: 10.1136/bmj.m1872
Marjanovic, Z., Greenglass, E. R., and Coffey, S. (2007). The relevance of psychosocial variables and working conditions in predicting nurses’ coping strategies during the SARS crisis: an online questionnaire survey. Int. J. Nurs. Stud. 44, 991–998. doi: 10.1016/j.ijnurstu.2006.02.012
Matias, G. P., Nicolson, N. A., and Freire, T. (2011). Solitude and cortisol: associations with state and trait affect in daily life. Biol. Psychol. 86, 314–319. doi: 10.1016/j.biopsycho.2010.12.011
Maunder, R., Hunter, J., Vincent, L., Bennett, J., Peladeau, N., Leszcz, M., et al. (2003). The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ 168, 1245–1251.
Mavrikaki, M., Pantano, L., Potter, D., Rogers-Grazado, M. A., Anastasiadou, E., Slack, F. J., et al. (2019). Sex-dependent changes in miRNA expression in the bed nucleus of the stria terminalis following stress. Front. Mol. Neurosci. 12:236. doi: 10.3389/fnmol.2019.00236
Mazza, M., Marano, G., Lai, C., Janiri, L., and Sani, G. (2020). Danger in danger: interpersonal violence during COVID-19 quarantine. Psychiatry Res. 289:113046. doi: 10.1016/j.psychres.2020.113046
Mihashi, M., Otsubo, Y., Yinjuan, X., Nagatomi, K., Hoshiko, M., and Ishitake, T. (2009). Predictive factors of psychological disorder development during recovery following SARS outbreak. Health Psychol. 28, 91–100. doi: 10.1037/a0013674
Murgatroyd, C., Patchev, A. V., Wu, Y., Micale, V., Bockmühl, Y., Fischer, D., et al. (2009). Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 12, 1559–1566. doi: 10.1038/nn.2436
Wuhan’s Coronavirus Outbreak: Life Inside the Quarantine. (2020). Washington, D.C: National Geographic.
Nord, A. S., and West, A. E. (2020). Neurobiological functions of transcriptional enhancers. Nat. Neurosci. 23, 5–14. doi: 10.1038/s41593-019-0538-535
Pellecchia, U., Crestani, R., Decroo, T., Van den Bergh, R., and Al-Kourdi, Y. (2015). Social consequences of ebola containment measures in liberia. PLoS One 10:e0143036. doi: 10.1371/journal.pone.0143036
Pournajafi-Nazarloo, H., Partoo, L., Yee, J., Stevenson, J., Sanzenbacher, L., Kenkel, W., et al. (2011). Effects of social isolation on mRNA expression for corticotrophin-releasing hormone receptors in prairie voles. Psychoneuroendocrinology 36, 780–789. doi: 10.1016/j.psyneuen.2010.10.015
Powell, N. D., Sloan, E. K., Bailey, M. T., Arevalo, J. M. G., Miller, G. E., Chen, E., et al. (2013). Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. PNAS 110, 16574–16579. doi: 10.1073/pnas.1310655110
Prem, K., Liu, Y., Russell, T. W., Kucharski, A. J., Eggo, R. M., Davies, N., et al. (2020). The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health 5, e261–e270. doi: 10.1016/S2468-2667(20)30073-30076
Punzi, G., Ursini, G., Viscanti, G., Radulescu, E., Shin, J. H., Quarto, T., et al. (2019). Association of a noncoding RNA postmortem with suicide by violent means and in vivo with aggressive phenotypes. Biol. Psychiatry 85, 417–424. doi: 10.1016/j.biopsych.2018.11.002
Reynolds, D. L., Garay, J. R., Deamond, S. L., Moran, M. K., Gold, W., and Styra, R. (2008). Understanding, compliance and psychological impact of the SARS quarantine experience. Epidemiol. Infect. 136, 997–1007. doi: 10.1017/S0950268807009156
Robertson, E., Hershenfield, K., Grace, S. L., and Stewart, D. E. (2004). The psychosocial effects of being quarantined following exposure to SARS: a qualitative study of Toronto health care workers. Can. J. Psychiatry 49, 403–407. doi: 10.1177/070674370404900612
Sandoval, A., Elahi, H., and Ploski, J. E. (2020). Genetically engineering the nervous system with CRISPR-Cas. eNeuro 7:ENEURO.419–ENEURO.419. doi: 10.1523/ENEURO.0419-19.2020
Scaccianoce, S., Del Bianco, P., Paolone, G., Caprioli, D., Modafferi, A. M. E., Nencini, P., et al. (2006). Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav. Brain Res. 168, 323–325. doi: 10.1016/j.bbr.2005.04.024
Schrempft, S., Jackowska, M., Hamer, M., and Steptoe, A. (2019). Associations between social isolation, loneliness, and objective physical activity in older men and women. BMC Public Health 19:74. doi: 10.1186/s12889-019-6424-y
Seebacher, F., and Krause, J. (2019). Epigenetics of social behaviour. Trends Ecol. Evol. 34, 818–830. doi: 10.1016/j.tree.2019.04.017
Serra, M., Pisu, M. G., Floris, I., Floris, S., Cannas, E., Mossa, A., et al. (2004). Social isolation increases the response of peripheral benzodiazepine receptors in the rat. Neurochem. Int. 45, 141–148. doi: 10.1016/j.neuint.2003.11.013
Shankar, A., Hamer, M., McMunn, A., and Steptoe, A. (2013). Social isolation and loneliness: relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosom. Med. 75, 161–170. doi: 10.1097/PSY.0b013e31827f09cd
Shankar, A., McMunn, A., Banks, J., and Steptoe, A. (2011). Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 30, 377–385. doi: 10.1037/a0022826
Siuda, D., Wu, Z., Chen, Y., Guo, L., Linke, M., Zechner, U., et al. (2014). Social isolation-induced epigenetic changes in midbrain of adult mice. J. Physiol. Pharmacol. 65, 247–255.
Spinelli, M., Lionetti, F., Pastore, M., and Fasolo, M. (2020). Parents’ stress and children’s psychological problems in families facing the COVID-19 outbreak in Italy. Front. Psychol. 11:1713. doi: 10.3389/fpsyg.2020.01713
Stankiewicz, P., Khan, T. N., Szafranski, P., Slattery, L., Streff, H., Vetrini, F., et al. (2017). Haploinsufficiency of the chromatin remodeler BPTF causes syndromic developmental and speech delay, postnatal microcephaly, and dysmorphic features. Am. J. Hum. Genet. 101, 503–515. doi: 10.1016/j.ajhg.2017.08.014
Stone, J., Evandrou, M., and Falkingham, J. (2013). The transition to living alone and psychological distress in later life. Age Ageing 42, 366–372. doi: 10.1093/ageing/aft006
Stranahan, A. M., Khalil, D., and Gould, E. (2006). Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 9, 526–533. doi: 10.1038/nn1668
Tabue Teguo, M., Simo-Tabue, N., Stoykova, R., Meillon, C., Cogne, M., Amiéva, H., et al. (2016). Feelings of loneliness and living alone as predictors of mortality in the elderly: the PAQUID study. Psychosom. Med. 78, 904–909. doi: 10.1097/PSY.0000000000000386
Taylor, M. R., Agho, K. E., Stevens, G. J., and Raphael, B. (2008). Factors influencing psychological distress during a disease epidemic: data from Australia’s first outbreak of equine influenza. BMC Public Health 8:347. doi: 10.1186/1471-2458-8-347
Thomas, E. Y., Anurudran, A., Robb, K., and Burke, T. F. (2020). Spotlight on child abuse and neglect response in the time of COVID-19. Lancet Public Health 5:e371. doi: 10.1016/S2468-2667(20)30143-30142
Thorsell, A., Slawecki, C. J., El Khoury, A., Mathe, A. A., and Ehlers, C. L. (2006). The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol. Biochem. Behav. 83, 28–34. doi: 10.1016/j.pbb.2005.12.005
How the Pandemic Could be Messing with your Sleep Time. (2020). Available online at: https://time.com/5858211/covid-19-sleep (accessed June 2020).
Verma, R., Harris, N. M., Friedler, B. D., Crapser, J., Patel, A. R., Venna, V., et al. (2016). Reversal of the detrimental effects of post-stroke social isolation by pair-housing is mediated by activation of BDNF-MAPK/ERK in aged mice. Sci. Rep. 6:25176. doi: 10.1038/srep25176
Verma, R., Ritzel, R. M., Harris, N. M., Lee, J., Kim, T., Pandi, G., et al. (2018). Inhibition of miR-141-3p ameliorates the negative effects of post-stroke social isolation in aged mice. Stroke 49, 1701–1707. doi: 10.1161/STROKEAHA.118.020627
Volden, P. A., Wonder, E. L., Skor, M. N., Carmean, C. M., Patel, F. N., Ye, H., et al. (2013). Chronic social isolation is associated with metabolic gene expression changes specific to mammary adipose tissue. Cancer Prev. Res. (Phila) 6, 634–645. doi: 10.1158/1940-6207.CAPR-12-0458
Wallace, D. L., Han, M.-H., Graham, D. L., Green, T. A., Vialou, V., Iñiguez, S. D., et al. (2009). CREB regulation of nucleus accumbens excitability mediates social isolation–induced behavioral deficits. Nat. Neurosci. 12, 200–209. doi: 10.1038/nn.2257
Wang, H.-T., Huang, F.-L., Hu, Z.-L., Zhang, W.-J., Qiao, X.-Q., Huang, Y.-Q., et al. (2017). Early-life social isolation-induced depressive-like behavior in rats results in microglial activation and neuronal histone methylation that are mitigated by minocycline. Neurotox. Res. 31, 505–520. doi: 10.1007/s12640-016-9696-9693
Wang, Y., Matz-Costa, C., Miller, J., Carr, D. C., and Kohlbacher, F. (2018). Uses and gratifications sought from mobile phones and loneliness among japanese midlife and older Adults: a Mediation Analysis. Innov. Aging 2:igy027. doi: 10.1093/geroni/igy027
Weaver, I. C. G., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., et al. (2004). Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854. doi: 10.1038/nn1276
Weber, J., Javelle, F., Klein, T., Foitschik, T., Crucian, B., Schneider, S., et al. (2019). Neurophysiological, neuropsychological, and cognitive effects of 30 days of isolation. Exp. Brain Res. 237, 1563–1573. doi: 10.1007/s00221-019-05531-5530
Weidner, M. T., Lardenoije, R., Eijssen, L., Mogavero, F., De Groodt, L. P. M. T., Popp, S., et al. (2019). Identification of cholecystokinin by genome-wide profiling as potential mediator of serotonin-dependent behavioral effects of maternal separation in the amygdala. Front. Neurosci. 13:460. doi: 10.3389/fnins.2019.00460
Weiss, I. C., Pryce, C. R., Jongen-Rêlo, A. L., Nanz-Bahr, N. I., and Feldon, J. (2004). Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res. 152, 279–295. doi: 10.1016/j.bbr.2003.10.015
Williams, J. B., Pang, D., Delgado, B., Kocherginsky, M., Tretiakova, M., Krausz, T., et al. (2009). A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prev. Res. (Phila) 2, 850–861. doi: 10.1158/1940-6207.CAPR-08-0238
Wu, P., Fang, Y., Guan, Z., Fan, B., Kong, J., Yao, Z., et al. (2009). The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can. J. Psychiatry 54, 302–311. doi: 10.1177/070674370905400504
Xu, S., Lu, J., Shao, A., Zhang, J. H., and Zhang, J. (2020). Glial cells: role of the immune response in ischemic stroke. Front. Immunol. 11:294. doi: 10.3389/fimmu.2020.00294
Yang, L., Engeland, C. G., and Cheng, B. (2013). Social isolation impairs oral palatal wound healing in sprague-dawley rats: a role for miR-29 and miR-203 via VEGF suppression. PLoS One 8:e72359. doi: 10.1371/journal.pone.0072359
Yao, B., Christian, K. M., He, C., Jin, P., Ming, G., and Song, H. (2016). Epigenetic mechanisms in neurogenesis. Nat. Rev. Neurosci. 17, 537–549. doi: 10.1038/nrn.2016.70
Yap, E.-L., and Greenberg, M. E. (2018). Activity-regulated transcription: bridging the gap between neural activity and behavior. Neuron 100, 330–348. doi: 10.1016/j.neuron.2018.10.013
Yatham, S., Sivathasan, S., Yoon, R., da Silva, T. L., and Ravindran, A. V. (2018). Depression, anxiety, and post-traumatic stress disorder among youth in low and middle income countries: a review of prevalence and treatment interventions. Asian J. Psychiatr. 38, 78–91. doi: 10.1016/j.ajp.2017.10.029
Yoon, M.-K., Kim, S.-Y., Ko, H.-S., and Lee, M.-S. (2016). System effectiveness of detection, brief intervention and refer to treatment for the people with post-traumatic emotional distress by MERS: a case report of community-based proactive intervention in South Korea. Int. J. Ment. Health Syst. 10:51. doi: 10.1186/s13033-016-0083-85
Zayed, A., and Robinson, G. E. (2012). Understanding the relationship between brain gene expression and social behavior: lessons from the honey bee. Ann. Rev. Genet. 46, 591–615. doi: 10.1146/annurev-genet-110711-155517
Zelikowsky, M., Hui, M., Karigo, T., Choe, A., Yang, B., Blanco, M. R., et al. (2018). The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell 173, 1265–1279.e19. doi: 10.1016/j.cell.2018.03.037
Zhu, F., Nair, R. R., Fisher, E. M. C., and Cunningham, T. J. (2019). Humanising the mouse genome piece by piece. Nat. Commun. 10:1845. doi: 10.1038/s41467-019-09716-9717
Zhu, J., Chen, Z., Tian, J., Meng, Z., Ju, M., Wu, G., et al. (2017). miR-34b attenuates trauma-induced anxiety-like behavior by targeting CRHR1. Int. J. Mol. Med. 40, 90–100. doi: 10.3892/ijmm.2017.2981
Zlatković, J., and Filipović, D. (2013). Chronic social isolation induces NF-κB activation and upregulation of iNOS protein expression in rat prefrontal cortex. Neurochem. Int. 63, 172–179. doi: 10.1016/j.neuint.2013.06.002
Keywords: non-coding RNAs, microRNA, long non-coding (lnc) RNAs, epigenetics, social isolation, behavior, COVID-19
Citation: Arzate-Mejía RG, Lottenbach Z, Schindler V, Jawaid A and Mansuy IM (2020) Long-Term Impact of Social Isolation and Molecular Underpinnings. Front. Genet. 11:589621. doi: 10.3389/fgene.2020.589621
Received: 31 July 2020; Accepted: 28 September 2020;
Published: 22 October 2020.
Edited by:
Bertrand Kaeffer, Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement (INRAE), FranceReviewed by:
Gopal Pandi, Madurai Kamaraj University, IndiaMurray John Cairns, The University of Newcastle, Australia
Copyright © 2020 Arzate-Mejía, Lottenbach, Schindler, Jawaid and Mansuy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabelle M. Mansuy, mansuy@hifo.uzh.ch
†Present address: Ali Jawaid, EMBL-Nencki BRAINCITY Center of Excellence for Neural Plasticity and Brain Disorders, Nencki Institute of Experimental Biology, Warsaw, Poland
 Rodrigo G. Arzate-Mejía
Rodrigo G. Arzate-Mejía Zuzanna Lottenbach
Zuzanna Lottenbach Vincent Schindler
Vincent Schindler Ali Jawaid
Ali Jawaid Isabelle M. Mansuy
Isabelle M. Mansuy